Introduction
For years, obesity has been framed as a consequence of excess eating and insufficient movement. Yet in clinical practice, this explanation repeatedly collapses. Individuals follow structured diets, restrict calories, exercise diligently, and still experience progressive weight gain, fatigue, intense hunger, and metabolic slowdown. These patterns reveal a deeper truth: obesity is rarely a behavioural issue first. It is a hormonal one.
Two hormones dominate this conversation that are insulin and leptin. Both are central regulators of energy storage, appetite, and metabolic flexibility. When either hormone stops communicating effectively with the body, fat loss resistance emerges. But a critical clinical question remains under-explored: which resistance comes first, insulin resistance or leptin resistance?
Understanding the sequence matters because it determines how obesity develops, why diabetes risk escalates, and why generic diet plans fail. At iThrive Alive, obesity and diabetes are approached as adaptive metabolic states driven by disrupted hormonal signalling, not as isolated diseases. This article examines insulin resistance vs leptin resistance through a systems biology lens, explaining how they interact, which tends to appear first, and how root-cause interventions restore metabolic intelligence.
Insulin Resistance | The Metabolic Traffic Jam
What Insulin Is Designed to Do
Insulin is a storage and signalling hormone released by the pancreas in response to rising blood glucose. Its primary job is to escort glucose into muscle, liver, and fat cells, where it can be used or stored for later energy. Under healthy conditions, insulin rises briefly after meals and falls during fasting, allowing metabolic flexibility between glucose and fat usage.
Insulin resistance develops when cells become less responsive to insulin’s signal. Glucose remains trapped in the bloodstream, prompting the pancreas to secrete even more insulin. Over time, this compensatory hyperinsulinemia becomes chronic, creating a metabolic environment biased toward fat storage and inflammation.
This is why insulin resistance and obesity are so tightly linked. Elevated insulin does not merely reflect weight gain, it actively promotes it by inhibiting fat breakdown and encouraging adipose expansion.
Why Insulin Resistance Develops Before Blood Sugar Rises
A critical misconception is that insulin resistance equals diabetes. In reality, insulin resistance can exist silently for years before fasting glucose or HbA1c rise. During this phase, the body is still compensating, but at a cost: worsening fatigue, abdominal fat gain, post-meal crashes, and increased hunger.
From a clinical perspective, insulin resistance is often the earliest metabolic disturbance in hormonal obesity. It alters fuel partitioning long before appetite hormones derail conscious eating behaviour.
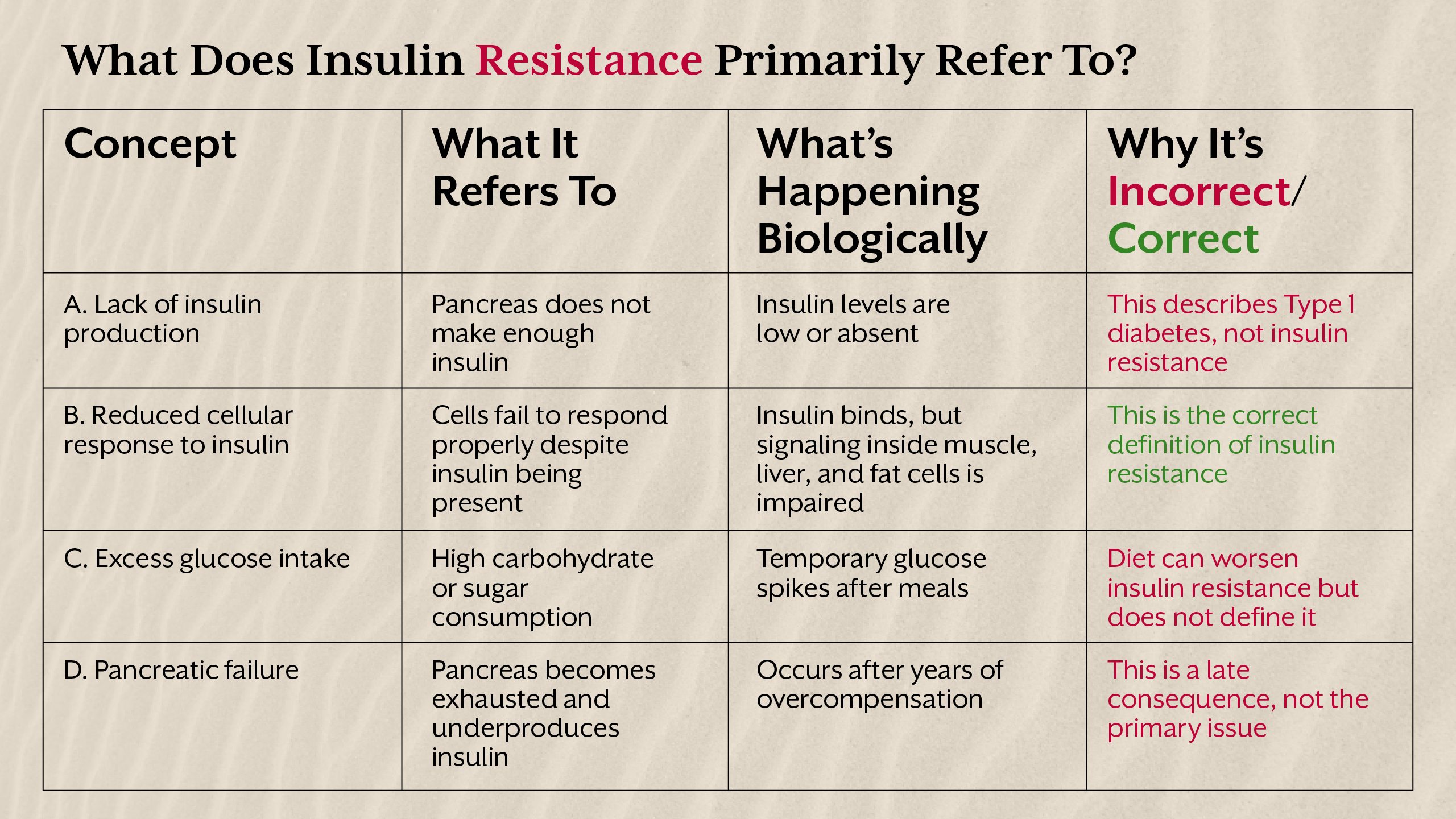
Leptin Resistance - When the Brain Stops Listening
Leptin’s Role in Energy Regulation
Leptin is secreted by fat cells and communicates with the hypothalamus to signal energy sufficiency. In a healthy system, rising fat stores increase leptin levels, reducing appetite and increasing energy expenditure. Leptin resistance occurs when the brain no longer responds appropriately to leptin’s signal, despite high circulating levels.
This is why individuals with obesity often have elevated leptin yet experience persistent hunger. Leptin resistance and obesity form a vicious cycle: fat gain increases leptin, but impaired signalling prevents appetite regulation.
Why Leptin Resistance Rarely Starts First
Contrary to popular narratives, leptin resistance is rarely the initiating defect. It typically emerges after prolonged metabolic stress such as chronic insulin elevation, inflammation, disrupted circadian rhythms, and poor sleep. Insulin directly influences leptin transport across the blood-brain barrier, meaning insulin resistance can impair leptin signalling upstream.
This sequence explains why appetite dysregulation often appears after years of weight gain, not before.
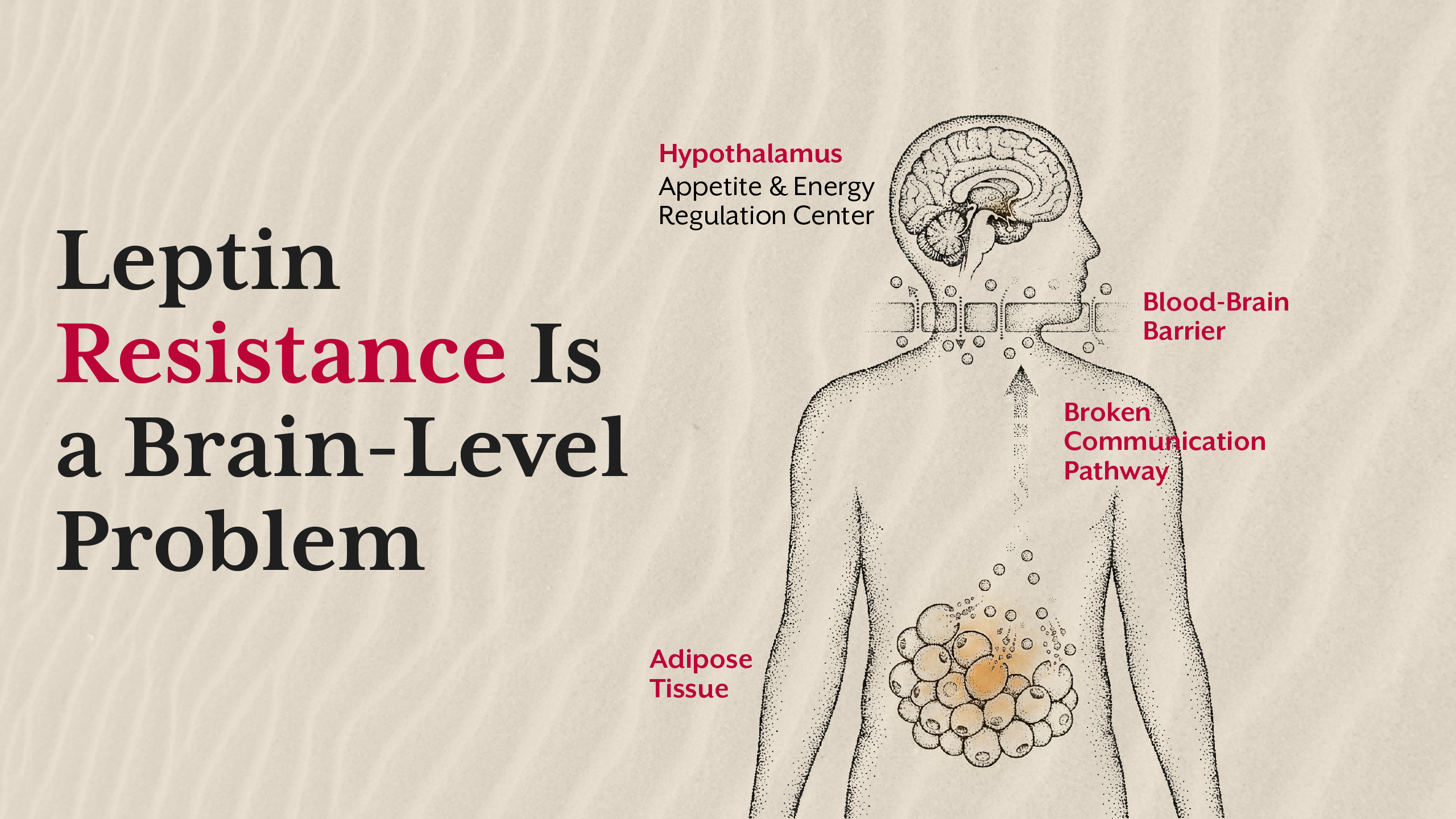
Insulin Resistance vs Leptin Resistance - The Clinical Sequence
In clinical observation, insulin resistance typically precedes leptin resistance. Elevated insulin alters fat storage patterns, promotes inflammation, and disrupts central appetite signalling over time. Leptin resistance then amplifies weight gain by removing the brain’s braking system.
This distinction matters because treating leptin resistance without addressing insulin resistance often fails. Appetite control strategies collapse when metabolic signalling remains impaired.
Fat Loss Resistance - When Biology Overrides Willpower
Fat loss resistance occurs when the body actively defends stored fat due to perceived metabolic threat. Chronic insulin elevation blocks lipolysis, while leptin resistance removes satiety cues. Together, they create a state where calorie restriction increases stress hormones, slows metabolism, and worsens outcomes.
This is why obesity and hormones must be addressed together. Without restoring signalling pathways, weight loss attempts reinforce the very adaptations driving weight gain.
The iThrive Alive Approach - Restoring Hormonal Intelligence
At iThrive Alive, obesity management focuses on reversing the drivers of insulin and leptin resistance through structured lifestyle interventions, targeted supplementation, and personalized eating strategies. Rather than suppressing symptoms, clinical reviews identify metabolic bottlenecks, circadian disruption, nutrient deficiencies, and inflammatory triggers.
Root-cause assessment allows insulin signalling to normalise first, creating the conditions for leptin sensitivity to recover naturally. Appetite stabilisation, energy improvement, and sustainable fat loss follow as outcomes, not goals forced through restriction.
Key Takeaway
Insulin resistance vs leptin resistance is not a debate of equals. Insulin resistance usually initiates the metabolic cascade that leads to leptin resistance, fat loss resistance, and obesity progression. Treating obesity without addressing this sequence leads to frustration and relapse. When hormonal communication is restored, weight regulation becomes a biological response rather than a daily struggle.
Subscribe to our newsletter and receive a selection of cool articles every week



.png)


.webp)

.jpg)
.jpg)