Introduction
Most conversations around obesity reduce overeating to a psychological weakness. The narrative is simple: stress leads to emotional eating, emotional eating leads to excess calories, and excess calories lead to weight gain. While emotions absolutely influence food behavior, this explanation is incomplete and often misleading.
At iThrive Alive, we frequently see individuals who insist they are “not overeating much,” yet weight continues to increase. Others describe relentless hunger, evening cravings, or an inability to feel satisfied even after a full meal. The assumption is often emotional fragility. The reality, more often than not, is metabolic imbalance.
Overeating in obesity is rarely just about comfort food. It is frequently the downstream effect of insulin resistance and obesity interacting with leptin resistance, disrupted satiety signaling, circadian misalignment, and cortisol-driven energy shifts. What appears as lack of discipline is often hormonal imbalance and weight gain unfolding in real time.
Understanding this distinction changes everything. Because if overeating is a signal of metabolic chaos rather than character weakness, the solution cannot be shame. It must be physiology.
The Biology of Hunger, More Than Just Willpower
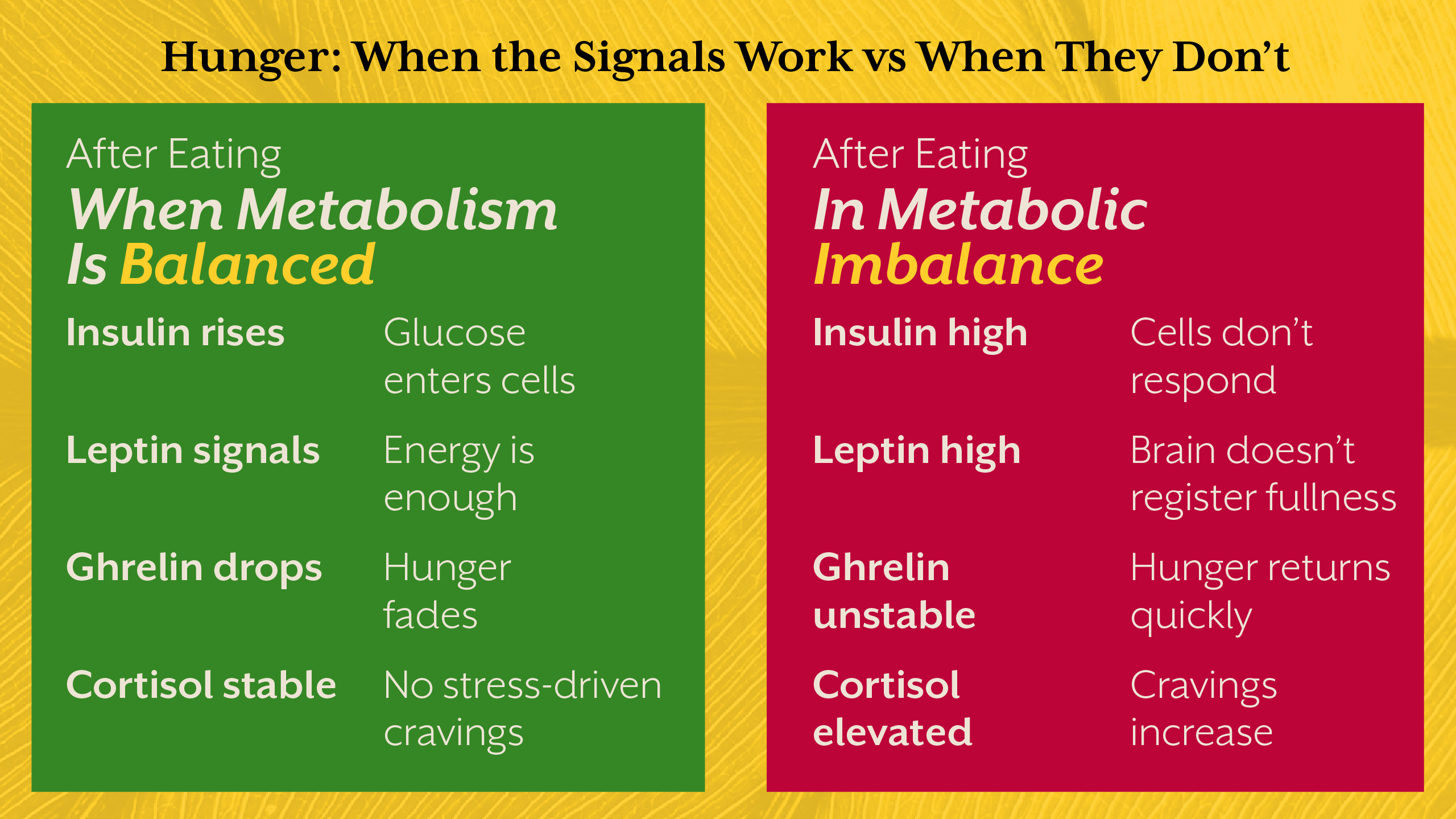
Hunger is regulated through an intricate network involving the gut, adipose tissue, pancreas, liver, and brain. Hormones such as insulin, leptin, ghrelin, and cortisol constantly communicate to determine whether we seek food or feel satisfied.
In a metabolically healthy individual, this system works with remarkable precision. After eating, insulin rises appropriately, glucose is stored or utilized, leptin signals energy sufficiency to the hypothalamus, and hunger naturally declines. Energy intake and expenditure remain balanced without conscious effort.
In metabolic imbalance, however, this regulatory loop begins to fail. Insulin resistance disrupts cellular energy access. Leptin resistance impairs the brain’s ability to sense stored fat. Cortisol alters glucose production and appetite patterns. The result is a body that behaves as if it is deprived, even when energy stores are abundant.
This is where overeating begins to shift from psychological narrative to biological survival response.
Insulin Resistance and Obesity, The Energy Lock Problem
One of the most overlooked drivers of overeating in obesity is insulin resistance. When cells become less responsive to insulin, glucose cannot efficiently enter muscle and liver tissue. Despite adequate energy intake, cells behave as though they are under-fueled.
This creates what we call an “energy lock.” Fat cells remain in storage mode, unable to release fuel effectively. The brain interprets this restricted fuel access as scarcity, triggering increased appetite.
In this context, overeating is not indulgence. It is a compensatory response to cellular energy dysfunction. This explains why individuals with insulin resistance and obesity often experience strong carbohydrate cravings or frequent hunger shortly after meals.
We explored the early compensatory phase of hyperinsulinemia in our blog, “Fasting Insulin vs Fasting Glucose: Which One Actually Predicts Type 2 Diabetes?” That silent elevation in insulin often precedes overt metabolic disease, and it also precedes relentless appetite dysregulation.
Leptin Resistance, When the Brain Stops Listening
Leptin is produced by fat tissue and signals satiety to the brain. In theory, higher body fat should mean stronger leptin signaling and reduced appetite. In obesity, the opposite frequently occurs.
Leptin resistance develops when the hypothalamus no longer responds adequately to leptin’s message. Despite high circulating leptin levels, the brain perceives energy insufficiency. Hunger remains elevated, and energy expenditure may decline.
This creates a paradox: more stored fat, yet persistent drive to eat.
In our earlier exploration of hyperinsulinemia as a hidden driver of metabolic dysfunction, we emphasized how hormonal feedback loops become distorted long before overt disease. Leptin resistance fits squarely within this broader metabolic imbalance narrative.
Overeating, in this case, is a brain-level communication failure, not emotional fragility.
Cortisol and Weight Gain - The Stress Amplifier
While insulin and leptin dominate the conversation, cortisol plays a crucial amplifying role. Chronic stress elevates cortisol, which increases hepatic glucose output and can promote insulin resistance over time.
Elevated cortisol also alters appetite regulation, often increasing cravings for energy-dense foods. Evening overeating patterns frequently correlate with disrupted circadian cortisol rhythms rather than emotional instability alone.
In this scenario, what appears to be “stress eating” is partially neuroendocrine recalibration gone wrong. Cortisol shifts fuel allocation, impairs sleep, and disrupts appetite hormones. The body attempts to restore stability through increased intake.
Understanding cortisol and weight gain reframes emotional eating as, in many cases, stress-driven metabolic adaptation.
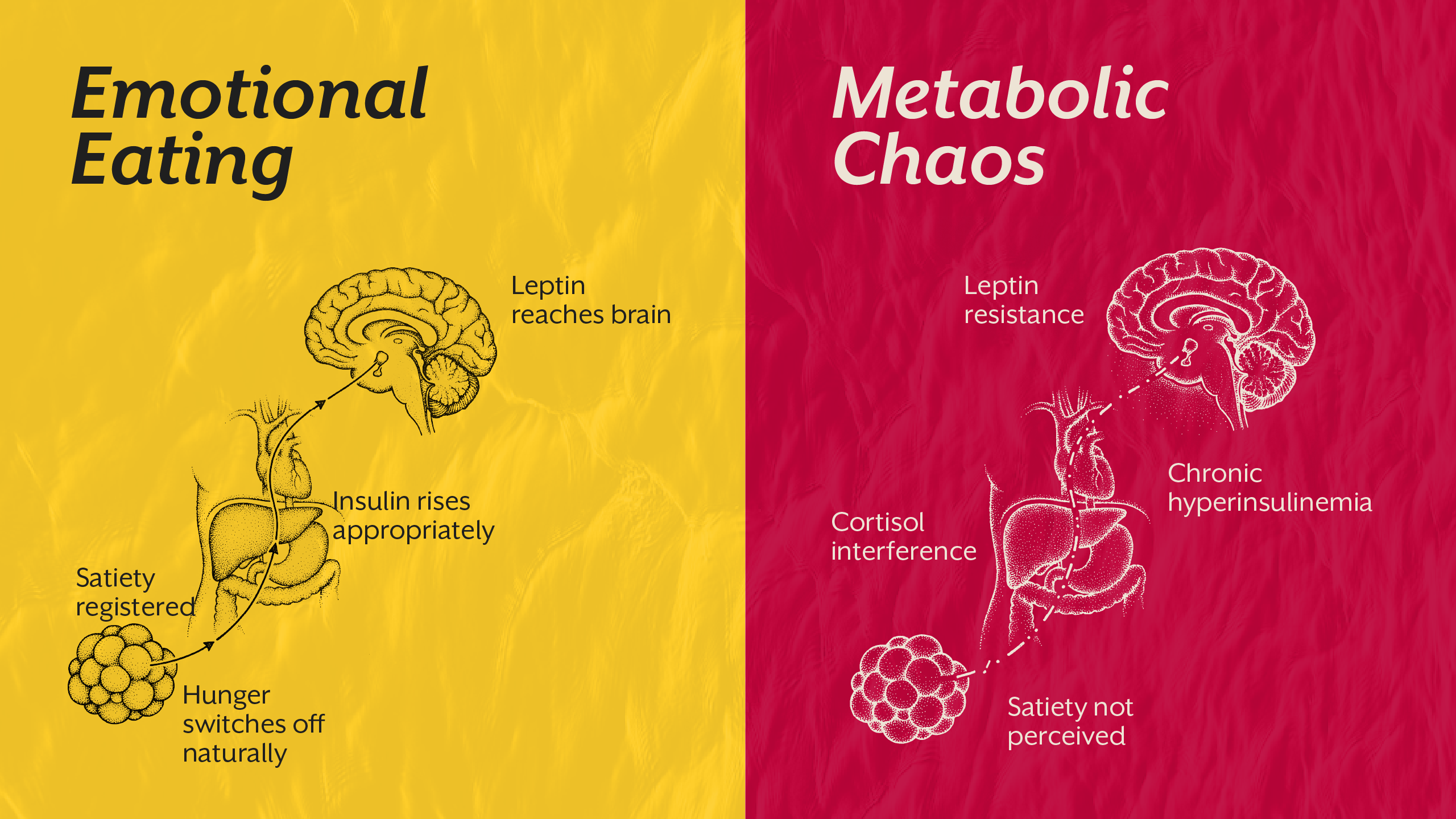
Emotional Eating vs Metabolic Chaos, How to Tell the Difference
Emotional eating certainly exists. However, not all overeating stems from emotional distress. Distinguishing between psychological triggers and physiological dysregulation is critical.
True emotional eating tends to be situational, episodic, and tied to specific emotional states. Metabolic-driven overeating is often persistent, characterized by strong hunger signals, reduced satiety, and physical symptoms such as energy crashes or sugar cravings.
At iThrive Alive, we rarely stop at surface narratives. We assess fasting insulin, inflammatory markers, lipid patterns, sleep rhythms, and stress physiology. Root cause analysis reveals whether overeating is primarily behavioral or hormonally driven.
Without this differentiation, interventions fail. Dieting strategies aimed at behavior alone cannot correct hormonal imbalance and weight gain driven by metabolic dysfunction.
The Role of Inflammation and Energy Sensing
Emerging obesity science highlights the role of low-grade inflammation in appetite dysregulation. Inflammatory cytokines can impair insulin signaling and disrupt hypothalamic appetite control.
Mitochondrial dysfunction further complicates energy sensing. When cellular energy production declines, the body increases hunger signals to compensate. This explains why some individuals overeat despite consuming calorie-dense diets, they are rather energy-replete but metabolically inefficient.
This deeper understanding of obesity and hormones challenges simplistic calorie-based explanations. Obesity is not merely about intake. It is about energy sensing and allocation gone awry.
Restoring Metabolic Balance - The iThrive Alive Approach
If overeating is driven by metabolic chaos, the solution must target underlying physiology. At iThrive, interventions focus on restoring insulin sensitivity, improving leptin signaling, recalibrating cortisol rhythms, and enhancing mitochondrial efficiency.
This involves structured meal sequencing, protein prioritization, micronutrient optimization, circadian alignment, and targeted supplementation. Smart eating is not restriction; it is strategic metabolic recalibration.
We also evaluate stress physiology, sleep architecture, and inflammatory load. Because addressing insulin resistance and obesity without correcting cortisol patterns or inflammatory triggers leads to incomplete recovery.
For individuals unsure whether their overeating is emotional or metabolic, a clinical review can clarify the drivers and guide precision intervention.
Obesity as a Signal, Not a Moral Failure
Perhaps the most important shift is conceptual. Obesity is not simply excess weight. It is a survival response to chronic metabolic stress.
When insulin resistance, leptin resistance, and cortisol dysregulation converge, the body prioritizes storage and hunger. Overeating becomes an adaptive attempt to restore perceived energy deficits.
Understanding this changes the emotional burden carried by individuals struggling with weight. The problem is not weakness. It is metabolic signaling gone wrong.
At iThrive Alive, this understanding shapes everything. We move beyond obesity as a behavior problem and treat it as a systems-level disorder requiring structured, physiology-first intervention.
Key Takeaway
Overeating in obesity is not always emotional fragility; it is frequently the outward expression of internal metabolic chaos. Insulin resistance locks energy inside cells, leptin resistance prevents the brain from recognizing stored fat, and cortisol reshapes appetite under chronic stress. Together, these hormonal disruptions create persistent hunger, cravings, and fat storage patterns that feel uncontrollable. When we shift the lens from willpower to physiology, solutions become clearer and more compassionate. True progress in obesity requires restoring metabolic balance, not merely suppressing appetite. At iThrive Alive, this root-cause perspective transforms overeating from a moral narrative into a solvable biological problem.
Subscribe to our newsletter and receive a selection of cool articles every week



.png)


.webp)

.jpg)
.jpg)